COHEAD
THERAPEUTICS
Accelerating treatment for cancer with covalent drugs
Unlocking the Undruggable with
AI-Powered Covalent Drug Discovery
CovAI
Platform
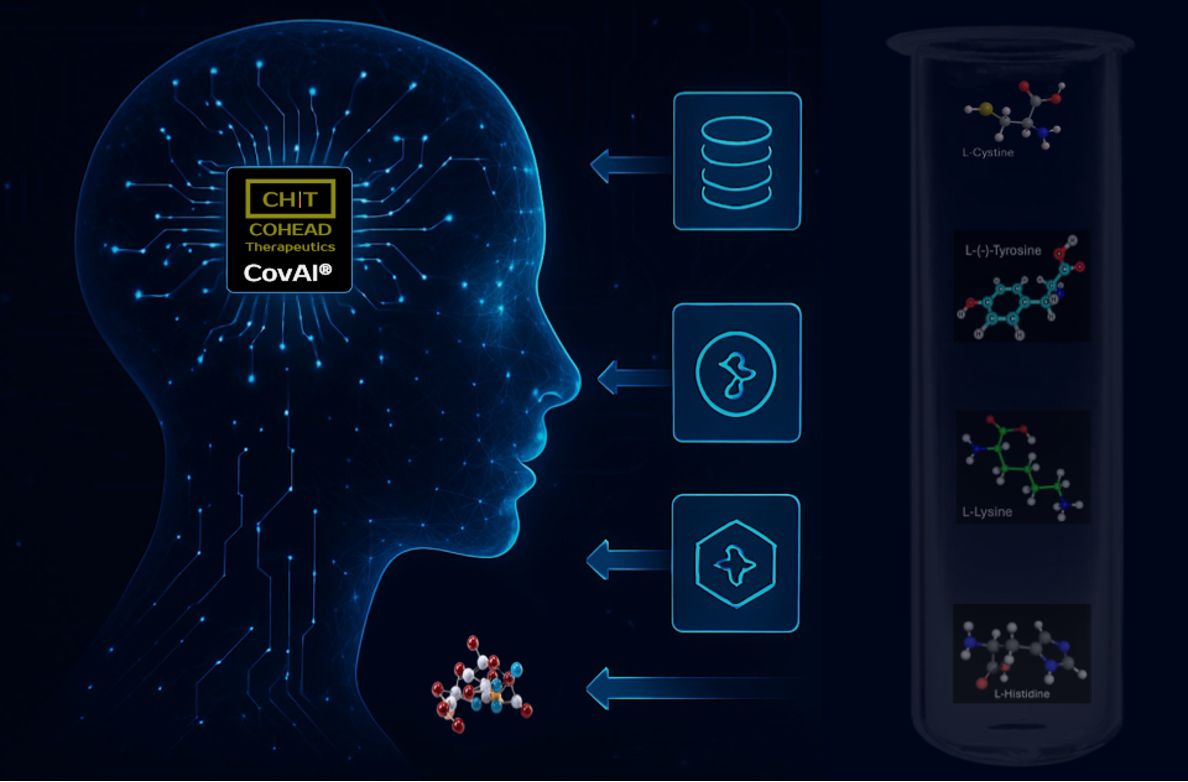
Advancing the covalent drug discovery beyond cysteine and the innovative CovAI platform in accelerating drug discovery.
We target proteins with no traditional binding pockets, using AI, quantum mechanics and molecular mechanics



Advancing the Covalent Drug Discovery Process
The COHEAD Breakthrough
Advancing the covalent drug discovery beyond cysteine and the innovative CovAI platform in accelerating drug discovery.
We expand the range of potential cancer drug targets by focusing on proteins without traditional binding pockets. Our approach improves selectivity, reduces toxicity, and enhances effectiveness.
By leveraging AI and quantum mechanics, we accelerate the drug discovery process and unlock novel therapeutic opportunities.
What are the covalent inhibitors?
Covalent inhibitors are revolutionizing drug discovery, offering unique advantages and significant therapeutic potential, particularly in cancer treatment. Unlike non-covalent inhibitors, covalent inhibitors selectively modify proximal nucleophilic amino acid residues on proteins, either reversibly or irreversibly, to achieve targeted and effective binding and therefore, covalent inhibitors can deactivate mutated proteins for extended periods, without extended toxicity. We have seen the power of covalent inhibitors through game-changing medications like Ibrutinib, Osimertinib and Sotorasib.
Historically, most successful covalent inhibitors have relied on binding site of cysteine residues. However, this approach has limitations for proteins lacking targetable cysteines. Recent advancements have focused on designing covalent inhibitors and probes targeting alternative proximal nucleophilic residues, such as lysine, tyrosine and histidine.
Why lysine targeted covalency in cancer?

Cysteine is drug developer's top choice for a covalent target as it’s high reactivity with covalent inhibitors and lysine is the second choice. Lysine is abundant across the proteome and present in the sequence of most protein drug pockets. At the physiological pH (7.4), the solvent exposed lysines can be protonated and will no longer make a covalent bond. Therefore, drug discovery efforts can go after lysines nestled within the inner folds of a protein, where they are protected from protonation. To explore the extent of lysine’s potential, researchers recently charted the landscape of lysines in human proteins to look for druggable sites. Their effort showed plenty of opportunities in this relatively unexplored space. At COHEAD Therapeutics, we have developed a unique innovative platform (CovAI) to identify reactive non-cysteine residues including lysine and optimize potent, selective covalent inhibitors with unparalleled speed and accuracy. This innovative approach streamlines and accelerates our hit-to-lead process, enabling us to tackle challenging targets and deliver impactful solutions for cancer therapy.
Expert Team & Scientific Advisors
World-Class Expertise Driving Breakthroughs
We have an ambitious, strong, creative, and diverse team of experienced scientists across medicinal, computational chemistry, cancer biology, and drug development.
Upul Bandarage
Co-Founder, President and Head of Research

Thushera Kawdawatta
CEO & Chief AI Officer

Kafi Belfon
Head of Computational Chemistry
Dr. Zachary Young
Head of Bilogy
Andre Pearson
Vice president, corporate development
Request a demo
Ready to see it in Action
AI-Driven Discovery Platform
Our CovAI Platform
The CovAI platform integrates multiple computational and experimental workflows, including machine learning-driven molecule selection, physics-based modeling, and reinforcement learning. These advanced techniques work together to optimize the discovery and refinement of covalent inhibitors.
Additionally, CovAI incorporates covalent binding validation using mass spectrometry and structural analysis. This comprehensive approach enhances the precision and efficiency of identifying, refining, and evaluating potential inhibitors.


Selective & Target Profiling
ABPP & CESTA combined with Physics based modeling & 3D ML approaches to understand and optimize selectivity.
Biophysical Techniques
Generate crystal structures with HDX-MX for modeling, SPR for binding affinity, and NMR/MS for covalent binding.

ADMET Prediction & Binding Free Energy
Filter molecules with good pharmacokinetics using ML models to optimize binding affinities.
QM Aware ML-Based Clustering
Select initial diverse molecules for docking from our large curated library of covalent inhibitors with Amino Acid specific warheads.
Covalent Docking with QM/MM Simulation
Identifies the best binders using QM based scoring functions that account for protein interactions and warhead reactivity

Reinforcement Learning (RL)
Select new molecules based on QM/MM scores to improve candidate via an iterative process.
REACH US
Connect with us
COHEAD Therapeutics opens to new partnerships and collaborations. Let’s work together to find novel medications for cancer
WHO WE ARE
Advancing the Covalent Drug Discovery Beyond Cysteine And Innovative CovAI Platform in Accelerating Drug Discovery
At COHEAD Therapeutics, we are redefining the targeted oncology landscape by designing first-in-class and best-in-class small molecule drugs. Our innovative approach uses non-cysteine targeting covalent inhibitors and reversible cysteine targeting covalent inhibitors, paving the way for new cancer treatments.
CovAI fuses AI-driven discovery with advanced computational chemistry to deliver powerful cancer therapies.
CovAI accelerates drug development using AI, quantum mechanic, molecular mechanics, and physics-based simulations.
Developing selective covalent inhibitors to tackle 'undruggable' cancer-drive proteins.
Uniting top-tier medicinal chemistry, computational biology, and AI/ML through a world-class team and academic partnerships.
Advancing five cutting-edge programs targeting key cancer drivers with novel covalent inhibitors.
Our History
COHEAD Therapeutics was founded in 2025 with the goal of developing new non-cystine targeting covalent inhibitors to improve the health of the patients with cancer.